EXPLORE: Matter
Physical Properties
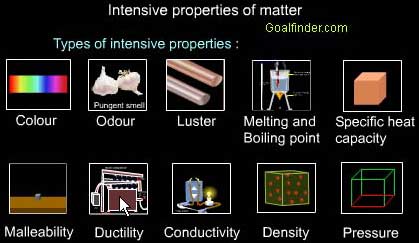
Matter has many properties. Some are physical properties. Physical properties of matter are properties that can be measured or observed without matter changing to a different substance. For example, whether a given substance normally exists as a solid, liquid, or gas is a physical property. Consider water. It is a liquid at room temperature, but if it freezes and changes to ice, it is still water. Generally, physical properties are things you can see, hear, smell, or feel with your senses.
Physical properties include the state of matter and its color and odor. For example, oxygen is a colorless, odorless gas. Chlorine is a greenish gas with a strong, sharp odor. Other physical properties include hardness, freezing and boiling points, the ability to dissolve in other substances, and the ability to conduct heat or electricity.
Examples of physical properties of matter. Image courtesy of CK-12.
Density is an important physical property of matter. It reflects how closely packed the particles of matter are. Density is calculated from the amount of mass in a given volume of matter, using the formula:
Problem Solving
Problem: What is the density of a substance that has a mass of 20 g and a volume of 10 mL?
Solution: 
To better understand density, think about a bowling ball and a volleyball. The bowling ball feels heavy. It is solid all the way through. It contains a lot of tightly packed particles of matter. In contrast, the volleyball feels light. It is full of air. It contains fewer, more widely spaced particles of matter. Both balls have about the same volume, but the bowling ball has a much greater mass. Its matter is denser.
LALALALALLALAA:ALL!KAZJOS)Z_WDKjn ecbxvdhjjy6ujimhn fbgrvf cdvgfvbgvfgehnbgvfcdfghnjhbgvfcdfvgbt
ReplyDeletesqdhcbxsajcdnfvmc dvngfcdxsz
ReplyDelete