Energy Transormations
Video Roller Coaster Video Build Your Own Roller Coaster More Links Brain Pop
Energy Transformation Virtual Lab Energy Transformation Game Bill Nye PK Energy
Bill Nye Advanced Build Space Ship Game Energy Transormations
Kinetic and Potential Energy Energy in Action
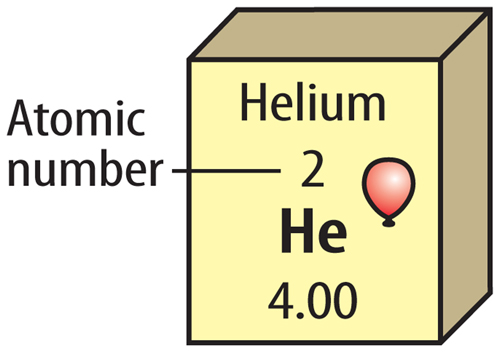
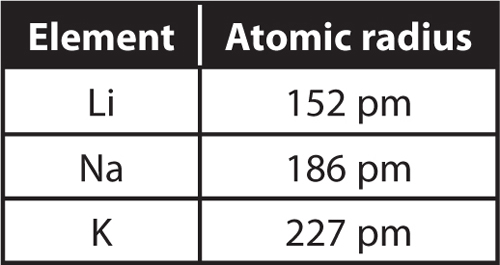
Just like there are different forms of electricity, there are different types of energy too. The two main types of energy are:

Other Types of Energy
There are other types of energy as well:
- Mechanical Energy is the energy of motion that does the work. An example of mechanical energy is the wind as it turns a windmill.
- Heat energy is energy that is pushed into motion by using heat. An example is a fire in your fireplace.
- Chemical Energy is energy caused by chemical reactions. A good example of chemical energy is food when it is cooked.
- Electrical Energy is when electricity creates motion, light or heat. An example of electrical energy is the electric coils on your stove.
- Gravitational Energy is motion that is caused by gravity. An example of gravitational energy is water flowing down a waterfall.
PE = mgh
where
- PE = Energy (in Joules)
- m = mass (in kilograms)
- g = gravitational acceleration of the earth (9.8 m/sec2)
- h = height above earth's surface (in meters)
KE = (1/2)mv2
where
- KE = Energy (in Joules)
- m = mass (in kilograms)
- v = velocity (in meters/sec)
Examples
For example, energy of food could be transformed in energy to play. Chemical energy from coal, oil, natural gas can be transformed into heat energy (process of burning the fuel). The heat energy can be converted into kinetic energy by gas turbines or into electrical energy by generators.
3 step energy transformation
 Sun gives the grass thermal energy. The grass use it to grow up and transform it to a chemical energy using the photosyntesis proces. Rabbit eat the grass and use it as an energy source to grow up and to have power to run.
Hunting dogs like rabits, but they can run after them only if they have anough power to do that. To have power dogs need to be feed.
2 step energy transformation
If you turn on the power switch the electricity bulb will use the electricity power and will transform in to light.
Sun gives the grass thermal energy. The grass use it to grow up and transform it to a chemical energy using the photosyntesis proces. Rabbit eat the grass and use it as an energy source to grow up and to have power to run.
Hunting dogs like rabits, but they can run after them only if they have anough power to do that. To have power dogs need to be feed.
2 step energy transformation
If you turn on the power switch the electricity bulb will use the electricity power and will transform in to light.

 The example of transformation of chemical energy into mechanical energy is the car engine.
The example of transformation of chemical energy into mechanical energy is the car engine.
Other example is the wind turbine that you know. Turbine uses the wind energy and transform in to electricity.
Solar panels transform light to electricity.
Look around you and try to find other examples for energy transformation.
For example, energy of food could be transformed in energy to play. Chemical energy from coal, oil, natural gas can be transformed into heat energy (process of burning the fuel). The heat energy can be converted into kinetic energy by gas turbines or into electrical energy by generators.
3 step energy transformation
 Sun gives the grass thermal energy. The grass use it to grow up and transform it to a chemical energy using the photosyntesis proces. Rabbit eat the grass and use it as an energy source to grow up and to have power to run.
Sun gives the grass thermal energy. The grass use it to grow up and transform it to a chemical energy using the photosyntesis proces. Rabbit eat the grass and use it as an energy source to grow up and to have power to run.
Hunting dogs like rabits, but they can run after them only if they have anough power to do that. To have power dogs need to be feed.
2 step energy transformation
If you turn on the power switch the electricity bulb will use the electricity power and will transform in to light.


Other example is the wind turbine that you know. Turbine uses the wind energy and transform in to electricity.
Solar panels transform light to electricity.
Look around you and try to find other examples for energy transformation.
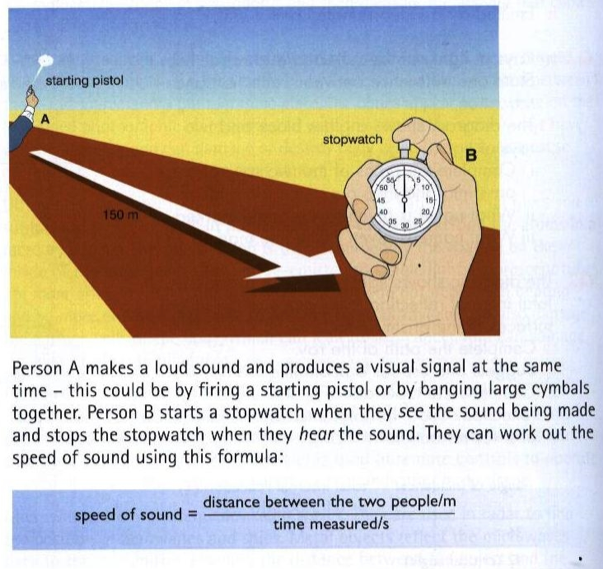
Now it's your turn
Create 5 word problems to calculate speed!
Create 5 word problems to calculate speed!